Today I did one of my favorite things at work. I got to do potions class. Here’s how I took ordinary tap water, made Felix Filicis, the Draught of Living Death, and then back to water.
(Note: the real ingredients and end product are actually very dangerous and should not be touched with your bare hands or drunk)
So first I started with plain old water, which must be rushing from the tap when you fill the bottle.
I added some powdered moonstone (Manganous sulfate) and crushed scarab (alkaline iodide-azide reagent). This turns the water into an orange floc which settles eventually.
To finish the Felix Felicis spell, you must add the dust of a fallen star (sulfamic acid powder) to the floc.
But the magic isn’t over. Next up was to make the Daught of Living Death, a potion you certainly don’t want to mess with.
First I transferred my Felix Felicis to a new flask. Then, of course, I need to add some valerian roots and the juice of the sopophorous bean (starch indicator). By now your Draught should be pretty dark.
 This is all pretty cool, but my favorite part comes next. Now’s when we take this deadly Draught and turn it back into water.
This is all pretty cool, but my favorite part comes next. Now’s when we take this deadly Draught and turn it back into water.
To do that, you need the liquid from a phoenix egg (sodium thiosulfate in liquid form). You drip this slowly into the flask until it turns blue…
…and lighter blue…
…and then, finally, until it turns clear as water once more.
And in real life, this will tell you the dissolve oxygen in your water. The magic trick as a whole is called the Winkler titration.
Hope you enjoyed today’s potions class. Tomorrow, perhaps, I will teach you how to find the antidote to an unknown poison (We call this a Toxicity Identification Evaluation in the lab).
Lots of love,
Sage




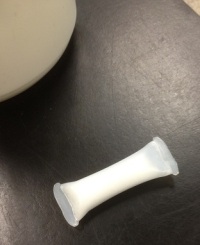
 Ta-dah! Felix!
Ta-dah! Felix!


This makes me so glad that I work with benign things like proteins and DNA. 🙂
I mean, the hardest part is getting the dust of the fallen star and the phoenix egg. You don’t use those at your lab?
hmm…dust of the fallen star – pollen? Phoenix egg – bovine serum albumin? haha… it looks totally cool though. The most color I get these days from a solution is with pH paper. 🙂
Haha, we just use a pH meter. The Winkler titration is to compare our dissolved oxygen meter to the titration as a monthly check. But in the field, I’ve done it as the DO reading.
We also titrate for hardness, but that’s not as cool (we don’t go from color to clear)
I’m not one for science, but if this was offered under Potions Class, I have to admit I’d take it up in a heartbeat. 🙂